Abstract
Refractory disease remains a challenge in treating patients (pts) with MDS and AML. Despite their widespread use, cytotoxic agents (7+3, HiDAC) and hypomethylating agents (HMAs) fail in the majority of pts, and lenalidomide (LEN) fails in 75% of non-del(5q) MDS. 90% of MDS and AML pts harbor at least one somatic mutation, further contributing to disease complexity. Currently, no comprehensive method exists to predict disease response based on the entire mutational burden detected in malignant samples. Predicting response to treatment would improve effectiveness, limit treatment-related adverse events, and reduce health care costs. Hence, there is need to predict pt response based on disease biology.
Aim: Determine the biological and clinical predictive values of a genomics-informed computational biology method (CBM) in pts who are treated with standard of care (SOC) therapy.
Methods: Pts with AML or MDS were recruited in a prospective clinical trial (NCT02435550) designed to assess predictive values by comparing computer predictions of treatment response to actual clinical response. Genomic profiling was conducted by cytogenetics, whole exome sequencing (WES), and copy number variation (CNV) analysis. These genomic results were entered into a CBM software (Cellworks Group), which generates disease-specific protein network maps using PubMed and other online resources. Digital drug simulations were conducted by quantitatively measuring drug effect on a cell growth score, which is a composite of cell proliferation, viability and apoptosis. Each pt-specific protein network map was digitally screened for the extent to which each pt's therapy reduced simulated disease growth in a dose-respondent manner. Treatment was physician's choice of SOC. Before initiating treatment, treating physicians were masked to the results of WES and CBM predictions. Clinical outcomes were prospectively recorded by the physicians. To be eligible for efficacy assessment, MDS pts must have had at least 4 cycles of HMA treatment or 2 cycles of LEN treatment. For AML, CR+PR was used to define response (IWG 2003). For MDS, CR+PR+HI was used to define response (IWG 2006). AML pt response was determined at time of recovery bone marrow biopsy, post induction therapy and/or consolidation therapy. Western blot assays were performed to validate the predicted protein network perturbations. Comparisons of CBM versus actual responses were performed using 2x2 tables. Fisher's exact tests were used to compare prediction values of the genomics-informed computer method versus empiric drug administration.
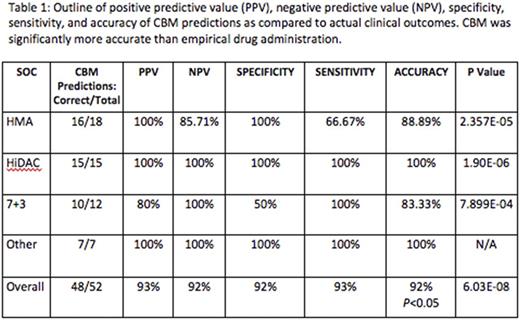
Results: Between June 2015 and July 2017, 120 pts were recruited, and 81 have had all genetic tests performed. At the time of this report, 42 pts were eligible for clinical response evaluation. 25/42 (60%) had AML, 15/42 had MDS (36%), and 2 pts had myelofibrosis. The median age was 65 (range 47-90). 24/42 (57%) were treatment-naïve and 18/42 (43%) were treatment-refractory. Laboratory validation study of computer-predicted, activated protein networks in 19 samples from 11 different pts showed correct prediction of 4 activated networks (Akt2, Akt3, PIK3CA, Erk1/2) in 17 samples, exhibiting 89% accuracy. At the time of this report, 42/81 pts received 52 treatments and were eligible for efficacy evaluation. 28/52 treatments resulted in clinical response to SOC therapy, while 24/52 did not achieve clinical response. 48 drug outcome predictions (92%) were correctly matched to their actual clinical outcomes, and 4/52 were incorrectly matched, resulting in 93% PPV, 92% NPV, 93% sensitivity, and 92% specificity (Table 1). Of note, CBM performed accurately in both treatment-naïve and relapsed/refractory pts.
The accuracy of the CBM was significantly greater than empiric drug administration (p=6.034 E-08). Additionally, new genomic signature rules were discovered to correlate with clinical response after HMA or len.
Conclusions: CBM that models multiple genomic abnormalities simultaneously showed high predictive value of protein network perturbations and clinical outcomes after SOC treatments. The network method uncovered molecular reasons for drug failure and highlighted resistance pathways that could be targeted to recover chemosensitivity. This technology could also be used to establish eligibility criteria for precision enrollment in drug development trials, and will be used in upcoming precision medicine trials.
Singh: Cellworks Research India Pvt. Ltd: Employment. Radhakrishnan: Cellworks: Employment. Ullal: Cellworks Research India: Employment. Talawdekar: Cellworks: Employment. Sikora: Cellworks: Employment. Nair: Cellworks: Employment. Bhowmick: Cellworks: Employment. Abbasi: Cellworks Group Inc.: Employment. Vali: Cellworks Group Inc.: Employment. Norkin: Celgene: Honoraria, Research Funding. Cogle: Celgene: Other: Membership on Steering Committee for Connect MDS/AML Registry.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal